

We can provide formulation services to solve solubility issues utilizing methods such as amorphous solid dispersion, to produce high functionng APIs.
Although many new drug candidates show great promise in terms of efficacy, further development is limited due to their poor solubility. Globally, this trend is on the increase. Since 1999, our business offers various solutions to increase the solubility of poorly water-soluble drug by our spray drying technology.
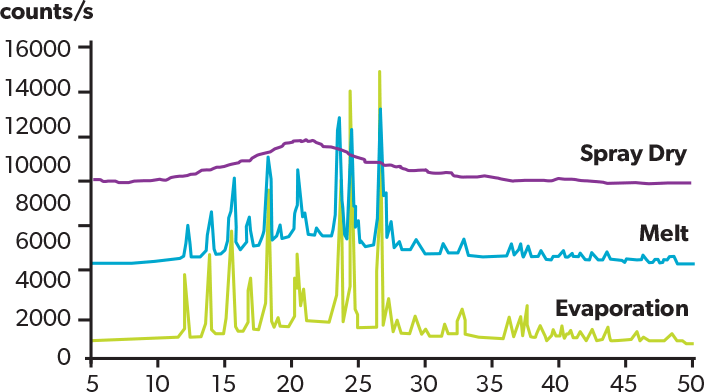
The figure shows XRD patterns of acetaminophen solid dispersion (acetaminophen: PVP = 1:0.35) prepared by spray dry, melting and evaporation methods. Only the spray dried product shows a halo pattern, indicating the spray dry method can produce amorphous solid dispersion with higher drug load.
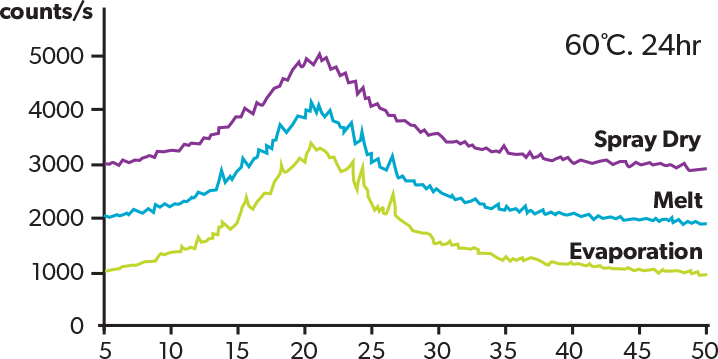
The XRD patterns of the acetaminophen solid dispersion (acetaminophen PVP = 1:1) obtained by different methods after being allowed to stand for 24 h at 60ºC are shown. All the curves show the halo pattern immediately after the manufacturing stage, but only the SD product exhibits the halo pattern at 24 h after manufacturing, indicating the advantage in stability over other methods.
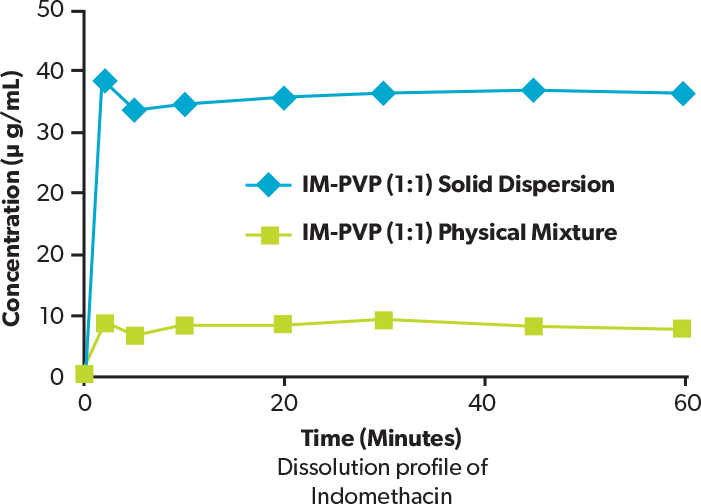
This graph shows the dissolution profile of indomethacin solid dispersion (indomethacin : PVP = 1 : 1). The apparent solubility of the indomethacin solid dispersion is about 6 times that of the physical mixture.
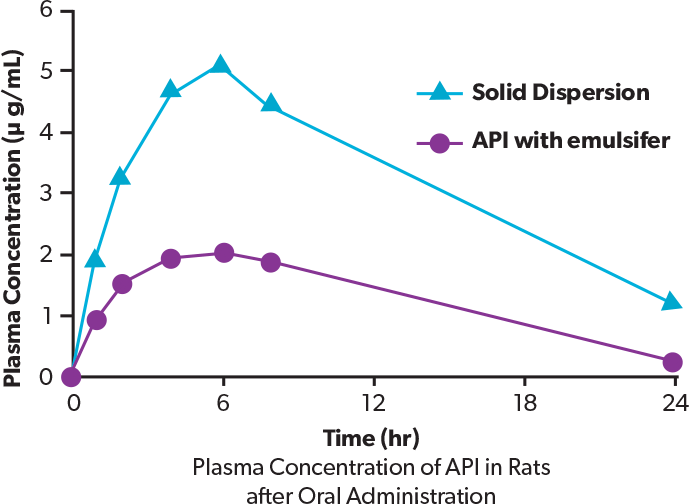
This graph shows the pharmacokinetic data obtained after administration of a compound A solid dispersion in rats. The AUC of the solid dispersion is higher than that of the pulverized product with emulsifier.
Since 1964, Fuji Chemical has provided contract manufacturing services to improve the powder properties of APIs and drug intermediates. To illustrate an example, we have developed processes to obtain various size powders or excellent molding property/tabletability by controlling the morphology of the spray dried particles. With our extensive experience and expertise in Spray Drying, we can address various customer needs concerning the powderization of APIs and improving powder properties.
We can convert any oily compound into powder by SD. Based on our original emulsion stabilization technology and knowhow of excipients, we can obtain high yields of powder containing high concentration of oily API. We have manufactured a number of oily APIs as well as health foods.